Welcome to STELFONTA®
STELFONTA®: An innovative and effective treatment for mast cell tumours in dogs
Complete
responseWound heals via
secondary intention*Rapid return of
quality of life
* Minimal intervention. Site of application should be covered for the first day after treatment to prevent direct contact with residual or leaking product.
STELFONTA® removes 75% of canine mast cell tumours with a single treatment*
87.2% of dogs had a complete response* after either one or two treatments combined**
RESPONSE
* Complete response was defined as complete removal of the tumour.Eisenhauer EA, et al. European Journal of Cancer 2009;45(2):228–47.
** Results from pivotal field efficacy study: 68/78 dogs achieved a complete response at 28 days after one or two treatments of STELFONTA®.
88% of dogs disease-free* at 12 monthsCampbell, Johannes, Reddell. Durability of clinical response to intratumoural tigilanol tiglate in canine MCT. Veterinary Cancer Society Conference 2019, Houston, Texas, USA.
FREE
74 dogs were available for assessment at 12 months following initial complete response to STELFONTA®. 65 dogs (88%) had no evidence of local MCT recurrence
* At the site of STELFONTA® treatment.
See STELFONTA® start to work within hours,Melo SR, et al. Veterinary Cancer Society, Houston, Texas, USA. with tumours typically destroyed by Day 7
-
![]()
Day 1 Acute inflammatory response with swelling and erythema to the tumour margins and immediate surrounding tissueMelo SR, et al. Veterinary Cancer Society, Houston, Texas, USA.
-
![]()
Day 7 Necrotic destruction is seen – in dogs this means blackening, shrinkage and leakage of thick discharge
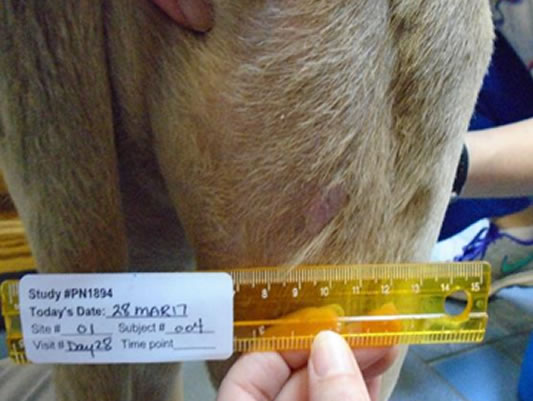
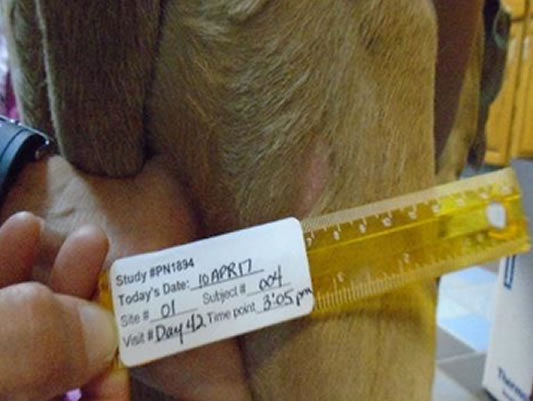
Hands-off healing* with more than half of tumour sites fully healed by Day 28
-
![]()
Day 28 56.5% wounds fully healed
-
![]()
Day 42 76.5% wounds fully healed
-
![]()
Day 84 96.5% wounds fully healed
Minimal intervention after treatment:
- Prophylactic antibiotic use not required
- Routine use of Elizabethan collars not required
* Minimal intervention. Site of application should be covered for the first day after treatment to prevent direct contact with residual or leaking product.
Dog owners report STELFONTA® treatment doesn’t negatively impact quality of lifeMiller J, et al JVIM 2020; article accepted in press.
“… By following the treatment process to the letter, Daisy coped exceptionally well, and it’s now 18 months later with no recurrence, and her quality of life is excellent. Daisy is now 10 and enjoying her later years with typical Jack Russell zest, energy and appetite!”
Brigitte and Brian Wright
Owners of Daisy

STELFONTA®’s story: Discovering nature’s natural defences
![]() DISCOVERY
DISCOVERY
STELFONTA® was discovered by Australian company QBiotics, who found a new biologically active chemical (tigilanol tiglate) in the seed of the Australian native blushwood plant (Fontainea picrosperma).
EMA-approved indications
STELFONTA® is an EMA-approved prescription treatment indicated for:
- All grades of non-metastatic mast cell tumours
- Mast cell tumours that are non-resectable
- All cutaneous mast cell tumours
- Subcutaneous mast cell tumours located at or distal to the elbow or the hock in dogs
Tumours must be less than or equal to 8 cm3 in volume, and must be accessible to intratumoural injection.
Important safety information
Formation of wounds is an intended reaction to treatment with STELFONTA®.
Most common adverse events such as pain, injection site bruising, erythema, oedema, lameness in a treated limb and wound formation are related to STELFONTA's mode of action
For more information, please review the Summary Product Characteristics.