The Use of Ciclosporin and HCA and Canine Atopic Dermatitis
HISTORY AND CLINICAL EXAMINATION
 A 7-month-old, female, entire, Hungarian Wirehaired Vizsla presented with a three-month history of generalised scale and alopecia of the head and neck with severe pruritus and erythema of the ventral abdomen and feet. She had previously been treated with a 2% chlorhexidine and 2% miconazole shampoo, topical fusidic acid and betamethasone gel, oral chlorpheniramine and oral essential fatty acids. After no response to these medications, oclacitinib at a dose of 5.4 mg BID for two weeks then SID thereafter, was prescribed off-licence (due to the dog being less than 12 months of age). The oclacitinib appeared to be effective, bringing the pruritus score down from 10/10 to 2/10, however the alopecia and erythema remained constant and a papular rash developed on the ventrum. At this point, cefalexin was commenced at a dose of 20 mg/kg BID.
A 7-month-old, female, entire, Hungarian Wirehaired Vizsla presented with a three-month history of generalised scale and alopecia of the head and neck with severe pruritus and erythema of the ventral abdomen and feet. She had previously been treated with a 2% chlorhexidine and 2% miconazole shampoo, topical fusidic acid and betamethasone gel, oral chlorpheniramine and oral essential fatty acids. After no response to these medications, oclacitinib at a dose of 5.4 mg BID for two weeks then SID thereafter, was prescribed off-licence (due to the dog being less than 12 months of age). The oclacitinib appeared to be effective, bringing the pruritus score down from 10/10 to 2/10, however the alopecia and erythema remained constant and a papular rash developed on the ventrum. At this point, cefalexin was commenced at a dose of 20 mg/kg BID.
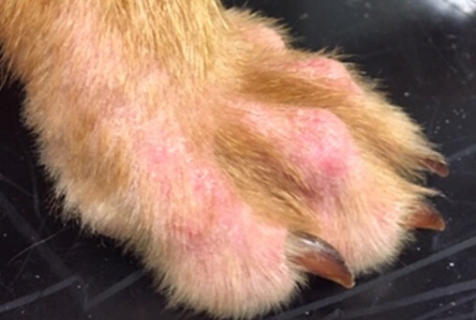
On presentation at the referral service, a full clinical examination was performed; there was a palpable lymphadenopathy with no other abnormalities on general physical examination. On dermatological examination, there was a generalised erythema, with a papular and macular eruption on the ventral abdomen. The feet were found to show alopecia, erythema and pruritus (figure 1).
DIFFERENTIAL DIAGNOSES
-
Ectoparasites
-
Sarcoptes
-
Fleas
-
Demodex
-
Lice
-
Cheyletiella
-
-
Infection
-
Malassezia spp. Overgrowth
-
Superficial bacterial folliculitis
-
Superficial pyoderma
-
-
Allergic skin disease
-
Flea allergic dermatitis
-
Cutaneous adverse food reaction
-
Canine atopic dermatitis
-
Contact allergy / irritant dermatitis
-
DIAGNOSTICS
Cytology samples were taken from the feet, ears and ventral abdomen to look for evidence of skin infection. These samples showed profuse numbers of Malassezia spp. in both ears and in the interdigital spaces of all four feet.
Skin scrapes were performed and examined for parasites; they were negative for demodex and sarcoptes. No visible parasites were found during an examination of the coat and a coat brushing yielded no evidence of flea or cheyletiella infestation.
An elimination diet trial was started with a commercial hydrolysed soy-based diet for eight weeks to allow exclusion of dietary allergens as a component of the cutaneous symptoms.

TREATMENT
 Cefalexin was continued at the same dose as existing prescribed, to treat the superficial pyoderma on the ventral abdomen. Bathing was continued with the 2% chlorhexidine and 2% miconazole shampoo. A gel comprising terbinafine, florfenicol and betamethasone acetate was applied to both ears, given the cytological findings consistent with a Malassezia spp. overgrowth. Oral oclacitanib and chlorpheniramine were discontinued from the treatment protocol due to a lack of efficacy in this case. The ectoparasite control regime was changed from imidacloprid and moxidectin monthly to sarolaner monthly to help rule out ectoparasites as a cause of the skin disease (sarolaner is licensed for both fleas and sarcoptes and some studies have shown that it has some efficacy against demodex).1
Cefalexin was continued at the same dose as existing prescribed, to treat the superficial pyoderma on the ventral abdomen. Bathing was continued with the 2% chlorhexidine and 2% miconazole shampoo. A gel comprising terbinafine, florfenicol and betamethasone acetate was applied to both ears, given the cytological findings consistent with a Malassezia spp. overgrowth. Oral oclacitanib and chlorpheniramine were discontinued from the treatment protocol due to a lack of efficacy in this case. The ectoparasite control regime was changed from imidacloprid and moxidectin monthly to sarolaner monthly to help rule out ectoparasites as a cause of the skin disease (sarolaner is licensed for both fleas and sarcoptes and some studies have shown that it has some efficacy against demodex).1
One week on, the owner found no reduction in the level of pruritus so oral prednisolone was added at a dose of 0.5 mg/kg SID for seven days then once every other day to reduce clinical signs whilst performing the diet trial. A spray of 0.584 mg/ml HCA, or hydrocortisone aceponate, was added to the treatment regime at this point to be applied topically to the feet.
Two weeks on, after tapering the prednisolone, the pruritus increased. The oral prednisolone was increased back to 0.5 mg/kg SID. The pruritus persisted despite a strict flea control programme, in the absence of infection and following a well adhered to eight-week food trial. This allowed a diagnosis of exclusion of canine atopic dermatitis (CAD) to be made. All skin infection and Malassezia spp. overgrowths had resolved with the treatment prescribed but the anti-inflammatory dose of prednisolone was not controlling the pruritus adequately. The decision was made to use liquid ciclosporin at a dose of oral 5 mg/kg SID.
Two further months on, the CAD has been well controlled with oral 5 mg/kg ciclosporin SID and topical 0.584 mg/ml HCA spray on the feet every other day. The erythema has resolved and the owner graded the pruritus at a sustained 2/10, which was an acceptable level, but without any of the other clinical signs (figure 2).
CONCLUSION
Canine atopic dermatitis (CAD) is a genetically predisposed condition where most dogs develop a Type I immunoglobulin E (IgE)-mediated hypersensitivity to environmental allergens. It is a very common disease, which certain breeds are predisposed to, but can occur in any dog.
CAD is chronic, relapsing and usually characterised by pruritus and inflammation; this can lead to secondary infections of the skin and ears. Clinical signs usually include erythema, saliva staining, alopecia, lichenification and hyperpigmentation, especially in those cases that have a chronic history. Signs of secondary infection, such as papules, pustules, epidermal collarettes and crusting can also be seen, depending on the severity.
To make the diagnosis, clinical signs and clinical history are very important, as there is no diagnostic test specific for AD. 2 Diagnostic criteria have been set out by Willemse et al. (1986), Prelaud et al. (1998) and more recently but Favrot et al. (2010). By Willemse’s criteria, at least three major and three minor features should be shown giving a sensitivity and specificity of 49.3% and 80.2% respectively.6 These criteria include:
-
Major factors –
-
Pruritus,
-
Chronic or relapsing dermatitis,
-
Individual / family / breed predisposition to atopy,
-
Facial / digital involvement or lichenification of flexor surface of carpus / tarsus.
-
-
Minor factors –
-
Onset less than three years,
-
Facial erythema or cheilitis,
-
Bacterial conjunctivitis,
-
Superficial staphylococcus spp. Pyoderma,
-
Hyperhidrosis,
-
Immediate positive intradermal test to inhalants,
-
Elevated allergen-specific IgE or IgGd.
-
In Prelaud et al. (1998), at least three of five signs should be shown with a sensitivity and specificity have been shown to be 74.3% and 68.4% respectively, with the signs being:
-
Corticosteroid responsive pruritus,
-
Pinnal erythema,
-
Bilateral erythematous pododermatitis,
-
Inflammation of the lips,
-
Appearance of first signs between six months and three years of age.
Favrot et al. (2010) proposed the following criteria with fulfilment of six of eight criteria giving a sensitivity of 85% and a specificity of 89% - this gives the highest sensitivity and specificity.6
-
Onset less than three years,
-
Mostly kept indoors,
-
Corticosteroid responsive dermatitis,
-
Chronic or recurrent yeast infections,
-
Affected front feet,
-
Affected ear pinnae,
-
Non – affected ear margins,
-
Non – affected dorso-lumbar area.
These criteria can be used as a very helpful aid in diagnosis, combined with appropriate diagnostics to rule out the other differentials.

1. Six, R., Becskei, C., Mazaleski, M., Fourie, J., Mahabir, S., Myers, M. & Slootmans, N. (2016). Efficacy of sarolaner, a novel isoxazoline, against two common mite infestations in dogs: Demodex spp. and Otodectes cynotis, Veterinary Parasitology, 222, p62-66.
2. Hensel, P., Santoro, D., Favrot, C., Hill, P. & Griffin, C. (2015). Canine atopic dermatitis: detailed guidelines for diagnosis and allergen identification, BMC Veterinary Research, 11, p196.
3. Willemse, T. (1986). Atopic skin disease: a review and a reconsideration of diagnostic criteria, Journal of Small Animal Practice, 27, p771-778.
4. Prelaud, P., Gauguere, E., Alhaidari, Z., Faivre, N., Heripret, D. & Gayerie, A. (1998). Re-evaluation of diagnostic criteria of canine atopic dermatitis, Revue de Médecine Vétérinaire, 149, p1057-1064.
5. Favrot, C., Steffan, J., Seewald, J., Seewald, W. & Picco, F. (2010). A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis, Veterinary Dermatology, 21, p23-30.
6. Miller, W.H., Griffin, C.E., Campbell, K.L. & Muller, G.H. (2013). Hypersensitivity Disorders. In: Muller and Kirk’s Small Animal Dermatology; Seventh Edition. St Louis: Elsevier.

