The Use of Ciclosporin as a Sole Agent in Canine Pemphigus Foliaceus
HISTORY AND CLINICAL EXAM
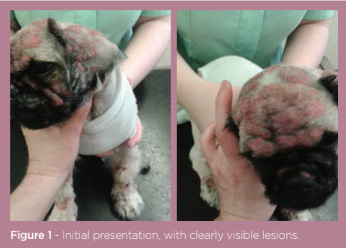 A nine-month-old, male, entire, Pekingese dog presented with an eruptive crusting and pustular dermatitis affecting the face, ears and dorsum. The dog was not pruritic but was dull and inappetent (figure 1). The dog had presented one week previously for erythema and pustules affecting only the facial folds; the initial therapy of topical 2% chlorhexidine and 2% miconazole shampoo and oral cefalexin at 15mg/kg BID proved ineffective; the dermatitis had progressed rapidly within this time.
A nine-month-old, male, entire, Pekingese dog presented with an eruptive crusting and pustular dermatitis affecting the face, ears and dorsum. The dog was not pruritic but was dull and inappetent (figure 1). The dog had presented one week previously for erythema and pustules affecting only the facial folds; the initial therapy of topical 2% chlorhexidine and 2% miconazole shampoo and oral cefalexin at 15mg/kg BID proved ineffective; the dermatitis had progressed rapidly within this time.
A full general clinical examination was largely normal but it did reveal a mild, generalised, peripheral lymphadenopathy. Dermatological examination revealed generalised hypotrichosis with erythema and discrete, coalescing, crusted pustules; this affected the head, ears, dorsum, flanks and distal limbs. The ventrum and mucous membranes were spared.
DIFFERENTIALS
-
Immune mediated disease
-
pemphigus foliaceus (PF)
-
pemphigus erythematosus
-
discoid and systemic lupus erythematosus
-
- Bacterial folliculitis
- Dermatophytosis (Trichophyton mentagrophytes)
- Demodicosis
- Cutaneous drug eruption
- Zinc responsive dermatosis
- Subcorneal pustular dermatosis
DIAGNOSTICS
Deep skin scrapings on microscopic examination were negative for ectoparasites. Wood’s lamp examination was negative for fungal infection and fungal culture of the hair and crusts yielded a negative result. Cytological examination of a ruptured pustule was stained with modified Wright’s stain revealed clusters of acantholytic cells and non-degenerate neutrophils. Histopathological examination of punch biopsy specimens, taken from four affected sites, indicated the presence of sub-corneal pustules, acantholytic cells and a combined neutrophil and eosinophil infiltrate. Fungal stains ruled out acantholytic dermatophytosis. Tissue culture yielded no aerobic or anaerobic growth.
Based on the clinical signs and a thorough dermatological diagnostic investigation, the dog was diagnosed with pemphigus foliaceus (PF).
TREATMENT
 Initially, immunosuppressive doses of oral prednisolone at 2mg/kg SID were initiated.
Initially, immunosuppressive doses of oral prednisolone at 2mg/kg SID were initiated.
Four weeks on, the owner reported the demeanour and appetite of the dog had improved. Many lesions had reduced in size and there were fewer pustules and crusts present. No new PF lesions were noted. However, generalised alopecia was notable with evidence of grease, scale and comedones, with the ventrum most affected. Deep skin scraping of these areas revealed high numbers of live juvenile and adult Demodex canis mites. Cytological examination of tape strips from affected areas showed profuse numbers of intra and extracellular coccoid bacteria. This was consistent with a diagnosis of generalised demodicosis and secondary bacterial pyoderma because of the immunosuppression from the prednisolone used to manage the PF.
Oral ciclosporin was initiated at 5mg/kg SID at the same time as prednisolone therapy was tapered over one week and then completely withdrawn. Amitraz was prescribed to treat the generalised demodicosis at the standard dose rate of 50ml concentrate per 5 litres of water. Cefalexin was initiated, as a good antibiotic choice in these cases, at 25mg/kg BID for the bacterial pyoderma.
The dog was monitored regularly. No new PF lesions were noted following initiation of ciclosporin treatment and withdrawal of prednisolone. Six weeks on, skin scraping was negative for Demodex canis mites and cytology indicated a resolution of the bacterial pyoderma thus oral cefalexin therapy was ceased. Ten weeks on, a subsequent second skin scraping was again negative for Demodex canis and the amitraz therapy was stopped. Twelve weeks on, the dog appeared in very good condition and the oral ciclosporin was tapered by 25% every 4 weeks until the treatment was completely withdrawn (figure 2). The dog currently remains in remission.
DISCUSSION
Pemphigus foliaceus (PF) is the most common bullous immune mediated dermatosis of dogs.1 Studies have shown a male bias and occasionally cases can occur at less than one year old, as seen here.2,3
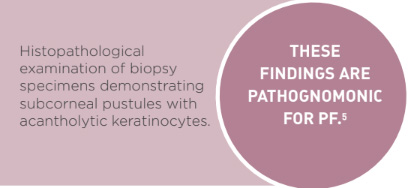
The clinical signs of pustules and crusts progressing from the head and ears to the dorsum, without mucous membrane involvement, is consistent with previous reports of the disease.1,4,5 Systemic signs of illness, including dullness, inappetence and generalised lymphadenopathy, are not a consistent finding.4 Diagnosis in this case was based on histopathological examination of biopsy specimens demonstrating subcorneal pustules with acantholytic keratinocytes; this is considered pathognomonic for PF.5
The most common therapy employed is immunosuppressive doses of prednisolone that may sometimes be combined with steroid-sparing agents, such as azathioprine or chlorambucil to minimise the effects of long-term glucocorticoids.4,6 Unfortunately, the development of severe generalised demodicosis with secondary bacterial pyoderma necessitated the complete withdrawal of glucocorticoid immunosuppressive therapy. Ciclosporin has been used successfully as a steroid sparing agent alongside anti-inflammatory doses of prednisolone to effect remission in canine PF.7 Rosenkrantz and Aniya (2007) demonstrated the effectiveness of ciclosporin as a sole agent to elicit and maintain remission in refractory cases of canine PF that failed to respond to a combination of prednisolone and azathioprine.
The case described here employed ciclosporin as a sole agent to manage PF which had become complicated by a secondary generalised demodicosis and bacterial pyoderma (due to the immunosuppressive oral glucocorticoid therapy). This suggests ciclosporin could be a powerful tool in the management of immune-mediated canine dermatoses, both by effecting and maintaining remission, but also by minimising the unwanted side effects caused by long-term glucocorticoid therapy.


