
How to use STELFONTA®
EMA-approved local treatment for non-resectable, non-metastatic mast cell tumours.
%20Stelfonta%20Indications%20image.png)
4 stages to treatment success with STELFONTA®
Prepare the tumour for STELFONTA® treatment with concurrent medications
- Mast cells contain granules holding histamine and heparin, as well as other chemical mediators
- Any manipulation of an MCT can lead to MCT degranulation reaction – this risk can be minimised by ensuring concurrent medications of corticosteroids, H1 and H2 receptor blocking agents are given as directed on label
- Corticosteroids are administered 2 days prior to the treatment, and continue daily until 7 days post-treatment
- H1 and H2 receptor blocking agents are started on the day of treatment and continue for 8 days
- Pain relief may be needed at the veterinary surgeons discretion (e.g Tramadol).
- Sedation or general anaesthesia should not be needed in most cases provided adequate restraint is possible

EXPERT TIP from
Dr. Justine Campbell
Don’t be tempted to skip the pre and concurrent medication; they’re both essential elements of the protocol and key to preventing potential systemic symptoms associated with MCT degranulation.
Concurrent medication dosing schedule
STELFONTA® injection
- Day
- Day
- Day
- Day
- Day
- Day
- Day
- Day
- Day
- Day
- Day
Potential period for clinical manifestation of degranulation
H1 and H2 receptor blocking agents
Corticosteroids
e.g. prednisolone; 0.5 mg/kg PO BID
Corticosteroids
0.5 mg/kg PO SID
Pain relief
May be needed at the veterinary surgeons discretion (e.g Tramadol).
BID: twice a day; SID: once a day; PO: by mouth.
Calculate the dose and inject STELFONTA® directly into the tumour
To administer STELFONTA®:
- Accurately measure the length, width and depth of the tumour (in cm)
- Determine the volume of the tumour:
Volume of tumour (cm3) = ½ x [length (cm) x width (cm) x depth (cm)] - Then determine the correct dose:
Dose (ml) = tumour volume (cm3) x ½
Using a sterile, Luer Lock syringe with a 23- to 27-gauge needle and wearing appropriate protective equipment (glasses and gloves), inject the dose by inserting the needle into the tumour mass through a single injection site, moving the needle in and out in a fanning manner to help ensure the treatment reaches all aspects of the tumour.



EXPERT TIP from
Dr. Justine Campbell
Measure and measure again – an accurate tumour measurement on the day of treatment (even if measured previously) is key to determining the correct dose.
Use a Luer Lock syringe to avoid leakage and potential bounce-back.
Generally speaking, I find if I can fine needle aspirate the patient without the need for sedation, I have no trouble administering STELFONTA® without a sedative.
Tumour breakdown is a sign STELFONTA® is working
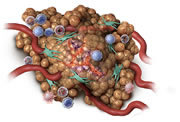
Oncolytic and inflammatory effect begins

Within hours: STELFONTA® starts to work, with changes visible at the tumour site. The cells in the tumour and tumour blood vessels will begin to break down resulting in a change of colour in the tumour and swelling at the treatment site.
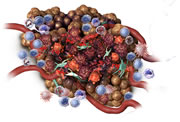
Increased permeability of tumour vasculature

Within days: The tumour may turn black. The surface may become soft and fluid may leak from the tumour as it continues to break down. Swelling at the treatment site may continue and cause some discomfort to the dog.

EXPERT TIP from
Dr. Justine Campbell
Prolonged presence of tumour tissue may slow the wound healing process, so it won’t hurt if a dog licks the wound.
Depending on the tumour size and amount of dead tissue, an odour may be present around Days 3 and 6 post-treatment.
Hands-off healing* via secondary intention with minimal intervention
- As the tumour breaks down further, a ‘pocket’ or deficit may develop where the tumour once was
- A healthy layer of new granulation tissue will be seen, indicating healing by secondary intention
- Site of application should be covered for the first day with a light dressing after treatment to prevent direct contact with residual or leaking product
- 56.5% of tumour sites healed by 28 days with 96.5% healed by day 84.


* Minimal intervention. Site of application should be covered for the first day after treatment to prevent direct contact with residual or leaking product.

EXPERT TIP from
Dr. Justine Campbell
If a scab or tumour tissue remains after Day 7, it can be removed if it is not attached to the underlying tissue, but it’s not necessary.
If a second treatment is required
- In clinical trials, 75% of tumours were removed with just one STELFONTA® treatmentWiest ML, Geller S, Pittenger ST, Burke-Schwarz C, Johannes, Chad M, Reddell PW, et al. Controlled, Randomised Study of Intratumoural Tigilanol Tiglate (EBC-46) for Treatment of Canine Mast Cell Tumours. In: ESVONC annual Congress proceedings, Frankfurt, 2019, p62.
- If visible tumour tissue remains 4 weeks after the initial treatment, the tumour should be remeasured and a new dose calculated for a second treatment.
Common adverse events are predicted by STELFONTA®’s MoA
- Most common adverse events such as pain, injection site bruising, erythema, oedema, lameness in a treated limb and wound formation are related to STELFONTA®’s mode of action at the tumour site
- Formation of wounds is a secondary intention to treatment with STELFONTA®
Expected adverse events seen following STELFONTA® treatment
- Signs due to mode of action include:
-
- Wound formation following tumour necrosis and slough
- Localised swelling, erythema, bruising at the tumour site
- Pain at the treated site
- Lameness in a treated limb
- Regional lymph node enlargement
- Signs from concurrent medication include:
-
- Polyuria
- Polydispsia
- Tachypnea
- Increased appetite
- Elevated alkaline phosphatase
- Signs of degranulation:
-
- Vomiting
- Diarrhoea
- Lethargy
- Anorexia/hyporexia
- Altered breathing/tachypnea
- Bruising and oedema at or away from the treated site
- Tachycardia
- Hypotension
For information on contraindications and warnings,
please refer to the Summary Product Characteristics



